West Pharmaceutical 2.0: Shifting from being just an elastomer supplier to providing integrated solutions

Introduction
West Pharmaceutical Services, Inc., founded in 1923 by Herman O. West, stands as a pioneering force in the global healthcare industry, with a rich history spanning nearly a century. Headquartered in Exton, Pennsylvania, West Pharma has evolved into a key player in providing innovative packaging components and delivery systems for injectable drugs. West has more than 10,000 employees across 50 sites worldwide, and supports customers by delivering approximately 47 billion components and devices each year.
From its early days manufacturing rubber components for injectable drug packaging, West Pharma has continually adapted to the evolving needs of the pharmaceutical and biotechnology sectors. Throughout its journey, the company has introduced groundbreaking technologies such as FluroTec® barrier film, the Flip-Off® seal, and the NovaGuard™ safety system. These innovations underscore West's position as a leader in developing critical components for drug delivery systems.
With a global footprint and a network of manufacturing facilities across multiple continents, West Pharma serves a broad spectrum of healthcare and pharmaceutical companies worldwide. The company's emphasis on quality, safety, and regulatory compliance has solidified its reputation as a trusted partner in the pharmaceutical packaging industry, which should continue to serve it well into the future.
Pharmaceutical packaging market
Traditionally, the pharmaceutical packaging industry is categorized into three segments: primary, secondary, and tertiary packaging. Primary packaging, or consumer packaging, directly interacts with drug products.
Therefore, understanding the potential interactions between primary pharmaceutical packaging containers and their contents is crucial. During the primary research and development stage, efforts are made to confirm drug product stability and compatibility to ensure that the packaging does not alter the chemical composition of the drug.
Secondary pharmaceutical contract packaging services involve packaging that consolidates individual units of primary packaged goods, often assembling them into multipacket units. Unlike primary packaging, secondary and tertiary packaging is not intended to hold the drugs but to facilitate their delivery in mass quantities to the point of sale or end user.
As you might have guessed, primary packaging is thus the most important of the three packaging categories. According to the latest regulation, call Annex 1 which was released in mid-2023, primary packaging has been defined as such “Primary packaging containers and components should be cleaned using validated processes to ensure that particulate, pyrogen and bioburden contamination is appropriately controlled.” The evolving regulatory landscape requires pharma and biotech companies to partner with a true expert who can help them navigate the new regulations successfully. Supplier components may be part of the overarching sterile manufacturing process and contamination control strategy; however, it is the responsibility of the finished product (drug/biologic) manufacturer to comply with the regulation.”
Now you might be wondering what are the different components that make up the primary packaging. These include:
- Medicinal product containers: bottles, pill bottles and dispensers in glass and plastic
- Pharmaceutical packaging closures: stoppers and plastic or aluminium caps
- Medical devices: drug delivery and dispensing accessories
There are two process to choose from when packing large molecule drugs in vials or syringes which need to be closed using a rubber stoppers and aluminium caps. These processes are required since injectable drug delivery bypasses the body’s natural barriers against bacteria and viruses, as such drugs delivered this way must undergo terminal sterilization or be produced via an aseptic process.
Terminal Sterilization or Aseptic fill-and-finish
Terminal sterilization is applied after drug manufacturing and involves heat, radiation, and/or filtration. However, this method isn’t always feasible, as it can negatively impact the product and its container. As drug formulations become more complex, a growing number of products cannot tolerate terminal sterilization and are sensitive to heat or radiation, reducing their effectiveness.
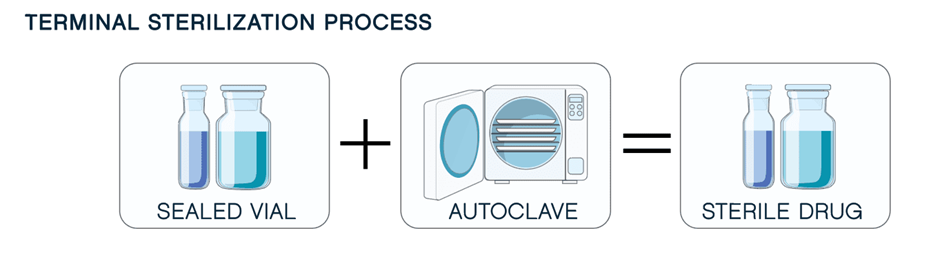
When terminal sterilization is not an option, aseptic manufacturing becomes the preferred approach. Aseptic Fill-Finish is a process in which the drug product, container, and container closure are first sterilized separately and then brought together. The step of combining the product, container and closure is done in a clean room and often uses special equipment that is self-contained in a sterile environment. When compared to the terminal sterilization, the aseptic fill-finish process significantly more challenging and complex.
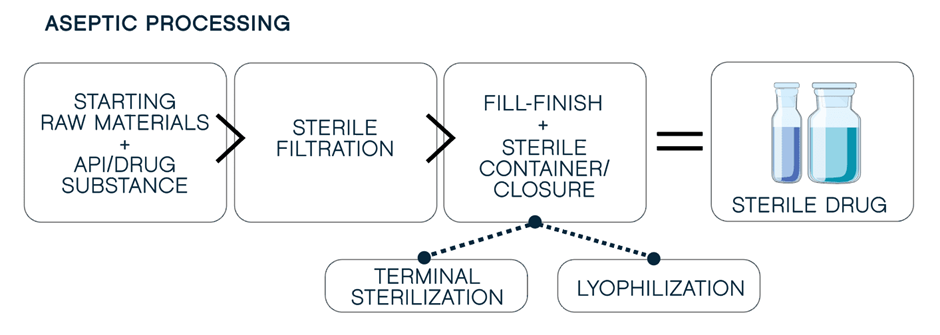
Aseptic Manufacturing & Sterile Fill-Finish Pharmaceutical Guide (pharm-int.com)
Fun Fact: For glass vials, although the vials are stoppered, the industry does not consider them closed until they have a cap crimped onto the system to make an integral container closure.
Keeping that in mind, let’s look at which segment West pharmaceutical plays in and makes money.




